Specific Heat Capacity Equation: A Comprehensive Guide
Specific heat capacity Equation is a fundamental property of matter that quantifies how much heat energy is required to raise the temperature of a unit mass of a substance by one degree Celsius. It is a measure of a substance’s ability to store and release heat.
The Formula for Specific Heat Capacity
The specific heat capacity (c) of a substance can be calculated using the following formula:
c = Q / (m * ΔT)
Where
Q is the heat energy transferred to or from the substance in joules, J
m is the mass of the substance in kilograms, kg
ΔT is the change in temperature of the substance (in degrees Celsius, °C)
Units of Specific Heat Capacity
The units of specific heat capacity are joules per kilogram per degree Celsius (J/kg°C). This means that it takes 1 joule of energy to raise the temperature of 1 kilogram of a substance by 1 degree Celsius.
Factors Affecting Specific Heat Capacity
Several factors can influence the specific heat capacity of a substance, including:
Molecular Structure: The arrangement of atoms and molecules in a substance can affect its specific heat capacity.
Pressure: The specific heat capacity of a gas can be affected by pressure.
Applications of Specific Heat Capacity
Specific heat capacity has numerous applications in various fields, including:
Engineering: In the design of heat exchangers, engines, and other thermal systems.
Climate Science: Understanding the specific heat capacity of the Earth’s oceans and atmosphere is crucial for climate modeling.
Materials Science: The specific heat capacity of materials is important in the development of new materials with specific properties.
Cooking: Knowledge of specific heat capacity can help in cooking and food preparation.
Examples of Specific Heat Capacity
Here are some examples of the specific heat capacities of common substances:
Water: 4186 J/kg°C
Copper: 385 J/kg°C
Aluminum: 900 J/kg°C
Iron: 448 J/kg°C
Gold: 129 J/kg°C
Specific Heat Capacity and Phase Changes
During a phase change e.g., from solid to liquid or liquid to gas), the temperature of a substance remains constant while heat is added or removed. This is because the energy is used to break or form intermolecular bonds, rather than increasing the kinetic energy of the molecules.
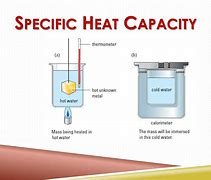
Calorimetry and Specific Heat Capacity
Calorimetry is a technique used to measure the specific heat capacity of a substance. It involves heating or cooling a known mass of the substance and measuring the temperature change and the amount of heat transferred.
Specific Heat Capacity and Energy Conservation
The law of conservation of energy states that energy cannot be created or destroyed, only transferred from one form to another. This principle applies to heat energy as well. The specific heat capacity of a substance determines how efficiently it can store and release heat energy.
Specific heat capacity is a fundamental property of matter that plays a crucial role in many areas of science and engineering. Understanding specific heat capacity can help us better understand the behavior of substances and improve the design of various systems and processes.
Faqs
What is specific heat capacity?
Specific heat capacity is a measure of how much heat energy is required to raise the temperature of a unit mass of a substance by one degree Celsius. It is a property of a substance that determines how easily it gains or loses heat.
What is the formula for specific heat capacity?
The formula for specific heat capacity is:
Q = m * c * ΔT
Where:
Q is the heat energy transferred in joules, J
m is the mass of the substance in kilograms, kg
c is the specific heat capacity of the substance in joules per kilogram per degree Celsius, J/kg°C
ΔT is the change in temperature in degrees Celsius, °C
How is specific heat capacity measured?
Specific heat capacity is typically measured using a calorimeter, a device that measures the heat gained or lost by a substance.
What is the difference between specific heat capacity and heat capacity?
Heat capacity is the amount of heat required to raise the temperature of a substance by one degree Celsius, regardless of its mass. Specific heat capacity is the heat capacity per unit mass.
What is the specific heat capacity of water?
The specific heat capacity of water is 4186 J/kg°C. This means that it takes 4186 joules of heat energy to raise the temperature of 1 kilogram of water by 1 degree Celsius.
Why does water have a high specific heat capacity?
Water has a high specific heat capacity due to its molecular structure and the strong hydrogen bonds between its molecules. This makes it difficult to break the bonds between water molecules, requiring more energy to raise its temperature.
What is the relationship between specific heat capacity and temperature change?
The specific heat capacity of a substance determines how much its temperature will change when a given amount of heat is added or removed. A substance with a high specific heat capacity will experience a smaller temperature change for a given amount of heat, while a substance with a low specific heat capacity will experience a larger temperature change.
How can specific heat capacity be used in everyday life?
Specific heat capacity is used in many applications, including:
Cooking: Understanding the specific heat capacity of different foods can help you cook them more efficiently.
Climate Control: The specific heat capacity of water plays a significant role in regulating the Earth’s climate.
Engineering: Specific heat capacity is used in the design of engines, heat exchangers, and other thermal systems.
What is the difference between specific heat capacity and thermal conductivity?
Specific heat capacity measures how much heat energy is required to raise the temperature of a substance, while thermal conductivity measures how quickly heat can be transferred through a substance.
How can I calculate the specific heat capacity of a substance?
You can calculate the specific heat capacity of a substance using the formula Q = m * c * ΔT. If you know the mass of the substance, the amount of heat added or removed, and the temperature change, you can solve for the specific heat capacity.
To read more click here











Post Comment